Therapeutic drug monitoring (TDM) is an approach for optimizing drug treatment regimens for individual patients based on the measurement of blood drug concentrations. Under the classical concept, TDM was done to avoid adverse events caused by drugs with narrow therapeutic windows that caused toxicities when given at even slightly elevated doses. TDM is applied in Japan for the immunosuppressants tacrolimus and cyclosporine, antiepileptics, and antiarrhythmics, and antimicrobials (e.g., aminoglycosides, glycopeptides, and the antifungal voriconazole). Ample evidence supports TDM for vancomycin used as first-line treatment for methicillin-resistant Staphylococcus aureus, which is a notable example of antimicrobial resistance. The concept of TDM has been evolving in recent years.
The International Association of Therapeutic Drug Monitoring and Toxicology (IATDMCT) offers the following definition of TDM: “TDM is a multi-disciplinary clinical specialty aimed at improving patient care by individually adjusting the dose of drugs for which clinical experience or clinical trials have shown it improved outcome in the general or special populations”1). The Japanese Society of Therapeutic Drug Monitoring defines TDM as “Pharmacotherapy individualized for patients while monitoring for factors related to therapeutic efficacy and adverse reactions”2). Notably, these definitions show that TDM is no longer done solely to avoid adverse reactions in individual patients and not necessarily limited to drugs with narrow therapeutic windows. This paper discusses the perspectives on antimicrobial TDM for infection treatment in emergency and intensive care settings. It focuses primarily on beta-lactam drugs, which were not considered in conventional TDM.
Beta-lactam drugs are widely used for their excellent safety profile and powerful bactericidal effects. Research is progressing on TDM of beta-lactam drugs with the goal of increasing survival in patients with infections. The defining antibiotic levels in intensive care unit patients (DALI) study by Roberts and colleagues showed that 16% of emergency/intensive care patients did not achieve adequate antibiotic exposure and may have benefited from higher doses3). The study also showed that blood concentrations of antibiotics varied markedly (Figure 1)3).
These findings mean that, if a fixed dose of an antimicrobial is to be increased as a strategy for reliably bringing the blood concentration of the drug within the therapeutic range, a high dose will have to be given to allow for patients who achieve the lowest concentrations. Figure 1 shows that, for the instance with the highest concentration, this would involve achieving a high concentration more than 60-fold that of the necessary concentration. Beta-lactam drugs, although safe, still pose risks to the central nervous system at extremely high doses. Beumier and colleagues defined a beta-lactam drug concentration exceeding 4 times the trough concentration / minimum inhibitory concentration (MIC) as a risk factor for neurotoxicity4). Encephalopathy is occasionally reported in association with cefepime and ceftriaxone5-7). Thus, though a certain number of patients in emergency and intensive care settings require high-dose beta-lactam treatment to survive, there was no clear way to safely increase the dose. TDM has emerged as a solution.
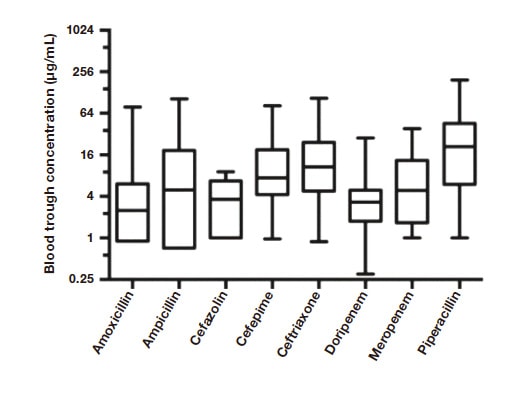
Fig. 1 Variation in blood trough concentrations of beta-lactam drugs in emergency/intensive care patients
The vertical axis in log scale shows the extent of the variation in trough values Source:Roberts JA, et al. Clin Infect Dis. 2014 Apr;58(8):1072-83.
European medical institutions are actively engaged in TDM for beta-lactam drugs. In 2019, The French Society of Pharmacology and Therapeutics and the French Society of Anaesthesia and Intensive Care Medicine released the world’s first guidelines on TDM for beta-lactam drugs8). The guidelines recommend TDM based on trough blood samples from 24 to 48 hours after the start of beta-lactam treatment. They also contain a flowchart showing a treatment design strategy based on concentration measurements (Figure 2). In the flowchart, the target concentration is from 4 to less than 8 times MIC, but it should be remembered that, as previously stated, concentrations exceeding 4 times MIC pose a risk of central nervous system adverse reactions. (Under these criteria, lower MIC values pose a lower risk of such adverse reactions.) The extent of the uptake of TDM for beta-lactam drugs in Europe was investigated in a 2016 study. In intensive care units in France, TDM was done for 30% of patients treated with ceftazidime, 21% of patients treated with piperacillin, and 19% of patients treated with meropenem9). In 2022, TDM for beta-lactam drugs given to patients on continuous renal replacement therapy was used in 45% of patients in university hospitals and 41% of patients in other types of hospitals, showing steady expansion of TDM10). Use of TDM in the Netherlands and Germany ranges from 15% to 30% for meropenem11,12).
As stated, TDM has been proposed as a strategy for reliably bringing antimicrobial drug concentrations into the therapeutic range to better save lives. TDM for beta-lactam drugs in Europe is now beyond its infancy and has entered a phase of broader adoption.
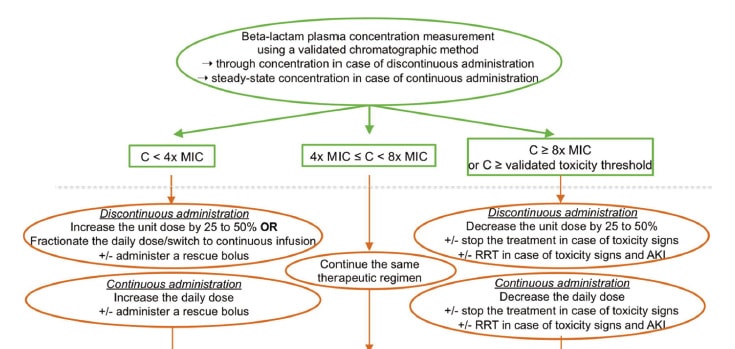
Fig. 2 Flowchart for adjusting dosages of beta-lactam drugs based on blood concentrations in TDM
The target concentration is from 4 to less than 8 times MIC, but the risk of neurotoxicity must be kept in mind.
Source:Guilhaumou R, et al. Crit Care. 2019 Mar 29;23(1):104.
No randomized, controlled trial (RCT) has shown that TDM enhances the therapeutic efficacy of beta-lactam drugs. The Dutch investigator Ewoldt and colleagues performed an RCT that included 388 intensive care patients administered beta-lactam drugs and ciprofloxacin who were assigned to treatment with or without TDM. TDM, however, failed to shorten ICU length of stay13). Blood concentrations in the non-TDM group (called the “standard dosing group” in the paper) showed a profile comparable to that of the TDM group (called the “MIPD group” in the paper). The investigators concluded that the study did not provide evidence to support TDM in patients with severe disease. Even so, given the nature of this study in general intensive care patients, it would be appropriate to find that beta-lactam TDM should not be recommended for all intensive care patients, and that investigations should proceed in a more limited group of patients.
Observational studies have investigated the effects on therapeutic efficacy of keeping the time the free antibiotic concentration is maintained above the minimum inhibitory concentration (%fT>MIC) at 50% or 100%3,14).
In summary, although there are no consensus target values for %fT>MIC for beta-lactam TDM, keeping values near these figures reportedly does affect therapeutic efficacy. To better demonstrate the effects of TDM on therapeutic efficacy, robust clinical studies limited to patients on TDM or with infections or in certain situations must be conducted. On this point, I believe that such RCTs are feasible because TDM for beta-lactam drugs is now used fairly widely in Europe. Such RCTs, however, are not feasible in Japan because beta-lactam TDM is not in use. Japan therefore lags Europe in beta-lactam TDM both in clinical practice and in studies. A solution is needed.
Blood concentration analyzers are key to beta-lactam TDM uptake. A 2022 survey of the status of hospital pharmacies by the Japanese Society of Hospital Pharmacists showed that drug concentration analysis was performed by pharmacies at 12.5% and by internal laboratories at 59.1% of the institutions surveyed15). These figures indicate that institutions have a substantial appreciation of TDM. However, with tests for beta-lactam drugs not covered under Japan’s national health insurance program, there is likely next to no assimilation of instruments capable of analyzing these drugs into clinical use. I contributed to joint research performed in the development of Hitachi High-Tech Science Corporation’s LM1010 drug concentration analyzer and have already clinically evaluated drugs including carbamazepine16), phenytoin16), lamotrigine, voriconazole17), meropenem, and vancomycin (Figure 3). The LM1010 is capable of measuring meropenem and other beta-lactam drug concentrations, in addition to those of many other drugs, and the drugs it can measure are being expanded.
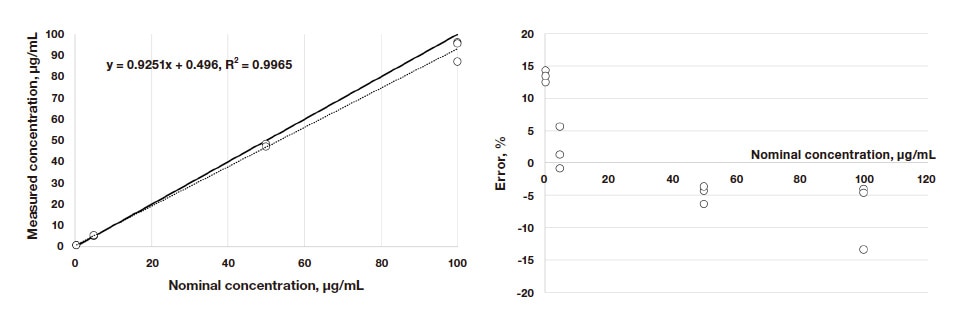
Fig. 3 Evaluation of meropenem concentration analysis
n=3 at the nominal concentrations of 0.5, 5, 50, and 100 μg/mL.
Left: Solid line is y=x, dotted line is regression line.
Right: Systematic error in Bland-Altman analysis. At the low concentration end, measured concentrations tended to deviate from nominal concentrations in the positive direction, but by no more than 15%. Accuracy is not an issue.
Indeed, the 2022 Guidelines for the Clinical Practice of Antimicrobial TDM propose using the area under the concentration time curve (AUC) on day 2 of treatment as a measure for treatment with vancomycin, which is a drug widely known to require TDM18). This proposal not only requires rapid action on day 2 of treatment, but also expertise regarding the AUC. These requirements essentially call for active involvement in TDM by pharmacy units. This addition to the Guidelines is prompting more medical institutions to work to do blood vancomycin concentration analyses internally rather than through contract laboratories. Institutions that purchase an LM1010 as they internalize analyses would gain a blood drug analyzer that is also able to measure beta-lactam drug concentrations.
TDM for beta-lactam drugs, whose blood concentrations vary substantially, most notably allows efficient dose adjustment to better save lives, as mentioned before. These efficiency gains will help caregivers not only reduce the risk of adverse events as they increase therapeutic efficacy, but also design special dosage regimens and reduce costs. Through journal articles and conference presentations, I am informing others about the patients who have benefited in my clinical practice of beta-lactam TDM in various forms.
A common cause of infections, P. aeruginosa, can defy treatment when it becomes drug-resistant, as it often does. Some of the drugs used to treat drug-resistant P. aeruginosa are highly toxic (e.g., colistin). When possible, safe betalactam drugs are a better option. We have treated patients with P. aeruginosa that was resistant to doripenem (a betalactam carbapenem), which has an MIC of 8 μg/mL. To avoid the need to use colistin, I used TDM to maintain the blood concentration of doripenem at 32 μg/mL, or 4 times the MIC. With my colleagues, I submitted a case report about the affected patient (Figure 4)19).
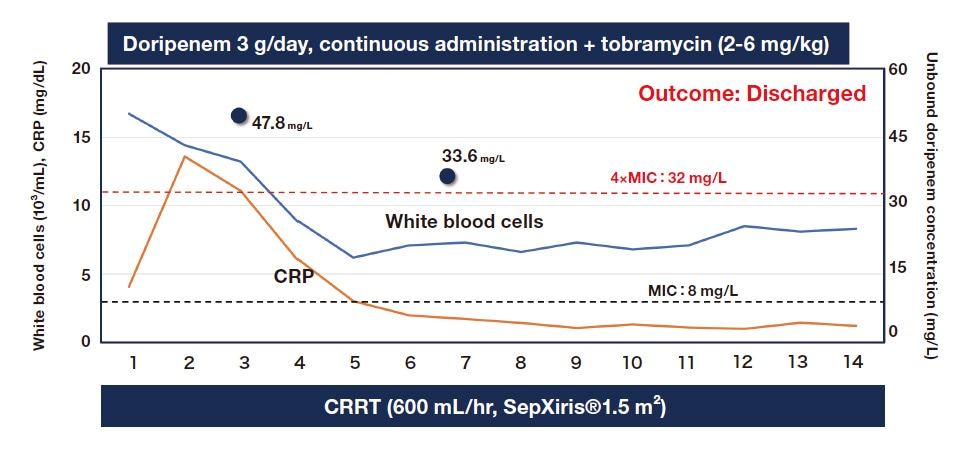
Fig. 4 TDM of doripenem to treat drug-resistant P. aeruginosa
Black circles indicate unbound doripenem concentrations in the serum.
CRRT: Continuous renal replacement therapy
Source:Oda K, et al. J Pharm Health Care Sci. 2019;5:15.
At excessive doses, cefepime causes encephalopathy5). TDM can be used to avoid this undesirable effect. I encountered a patient with aphasia who appeared to have cefepime-induced neurotoxicity. TDM showed a high cefepime concentration of 71.3 μg/mL. We would have considered switching to another drug but knew the patient had P. aeruginosa that had already become resistant to other drugs. We therefore decided to reduce the dose based on the pharmacokinetic analysis of cefepime. This approach cured the infection (Figure 5)20).
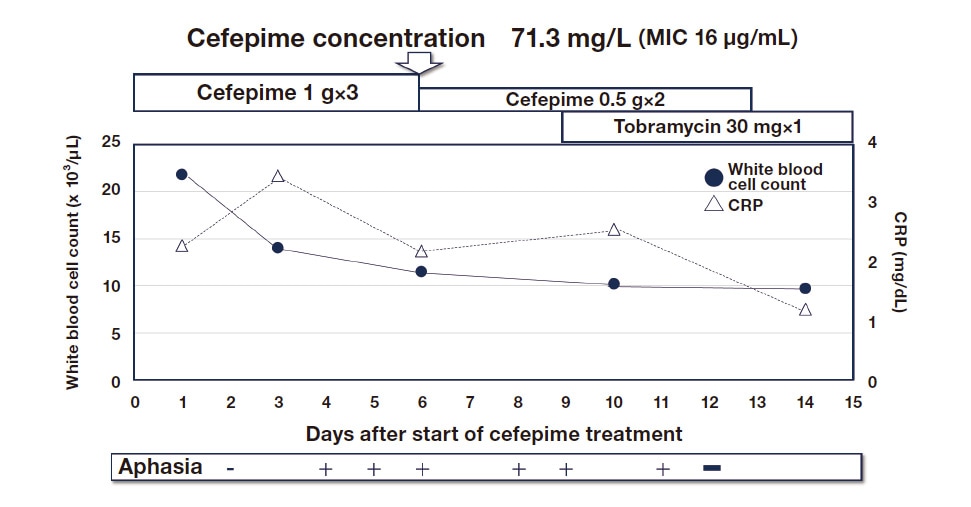
Fig. 4 Ongoing cefepime treatment for drug-resistant P. aeruginosa
TDM-based dose reduction of cefepime for neurotoxicity in this patient with aphasia cured the infection.
Source:Oda K, et al. J Infect Chemother. 2020;26(5):498–501.
Mediastinitis is a serious complication of cardiovascular surgery. Since information about antimicrobial uptake by the mediastinum is lacking, drugs must be selected with the utmost of care. A patient we treated had mediastinitis caused by an extended-spectrum beta-lactamase (ESBL)-producer. Since carbapenems are recommended as first-line treatment for infections by ESBL-producers, a carbapenem must be used in most instances. We, however, decided to continuously administer flomoxef and cefmetazole (which are known to be effective in mild urinary tract and other infections) while monitoring concentrations of the unbound forms of these drugs in the mediastinum and blood, thereby keeping the patient off carbapenems. The concentrations of the unbound drugs in the mediastinum were, relative to a target concentration of MIC ≤ 4 μg/mL, 10.6 μg/mL for flomoxef and 7.0 μg/mL for cefmetazole. These concentrations cured the infection.
We were involved in the treatment of a patient with P. aeruginosa pneumonia. The patient’s blood cultures remained positive for over 3 weeks despite treatment with the maximum dose of ceftazidime as an antibiotic to which the bacterium was sensitive. We determined the blood concentration to refer to just in case the drug concentration was too low and required switching to a carbapenem or other drug. P. aeruginosa remained sensitive because the MIC was 8 μg/mL. The patient, however, had extremely high trough and peak concentrations of 56 and 97.7 μg/mL. We told the team these concentrations were overkill. The dose was halved. The patient’s blood culture subsequently tested negative. In this case, avoiding unnecessary antibiotic use helped reduce costs.
TDM is demonstrably useful in beta-lactam treatment design. Through my clinical experience, I realize that TDM helps caregivers avoid giving patients overly broad antimicrobials by providing information about therapeutic efficacy, as I have discussed in the above cases involving the use of flomoxef and cefmetazole, ongoing use of cefepime, and dose reduction of ceftazidime. After all, reducing the doses of broad-spectrum antimicrobials used is a critical step in solving the problem of antimicrobial resistance. Since Japan’s national health insurance program does not cover beta-lactam TDM, personnel at most institutions will probably be hesitant to do beta-lactam TDM on a “volunteer” basis. However, given the high price of most broad-spectrum antimicrobials, avoiding their use, and avoiding high doses as in the above case involving ceftazidime, will help reduce costs for hospitals. In a future paper, I wish to show how beta-lactam TDM can increase profitability at individual medical institutions.
References
See more