Amino acids are a basic family of molecules in living organisms. With the exception of the irregular case of glycine, the carbon atom at the α site of each amino acid serves as an asymmetric center. Due to the presence of this asymmetric carbon, two inequivalent forms exist, known as enantiomers called D- and L-forms. Amino acids are believed to have been synthesized by chemical reactions in the primitive Earth, and these chemical reactions normally produce the two enantiomers in equal proportion, yielding a 50/50 blend of D-form and L-form molecules known as a racemic mixture.
However, the amino acids found in living organisms on Earth today are mostly L-forms, a phenomenon known as homochirality whose origin remains unclear. In the study of extraterrestrial samples, the Murchison meteorite, which fell in the Australian village of Murchison in 1969, was discovered to contain a variety of amino acids. The finding suggested the possibility that amino acids on Earth may have extraterrestrial origins. It was also reported that some of the amino acids contained in the meteorite were predominantly L-forms, and a hypothesis that the homochirality was itself of extraterrestrial origin was inspired. Therefore, accurate analysis of chiral amino acids in extraterrestrial samples is expected. However, extraterrestrial samples collected on Earth may be contaminated by some proportion of terrestrial amino acids (L-form rich), and this concern has motivated the planning of sample-return missions to collect samples from cosmic space and return them to Earth.
However, the test specimens available from extraterrestrial samples are limited, and many varieties of organic compounds are present in these samples due to radical reactions occurring in extraterrestrial space. Therefore, the development of analytical methods capable of quantifying chiral amino acids with high sensitivity and high selectivity is required. In this work we developed a multi-dimensional HPLC system for the accurate determination of chiral amino acids in many types of extraterrestrial samples, and applied it to the analysis of meteorites and asteroids.
In the extraterrestrial samples, we focus on the determination of five aliphatic amino acids shown in Figure 1: the proteinogenic amino acids alanine (Ala) and valine (Val), and the non-proteinogenic amino acids 2-aminobutyric acid (2AB), norvaline (Nva), and isovaline (Iva). These amino acids are heated for 2 min at 60°C in the presence of Na-borate buffer (pH 8.0) after adding 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F) to yield fluorescent derivatives. Then the reaction is terminated by adding an aqueous trifluoroacetic acid (TFA) solution and a portion of this reaction mixture is analyzed by a multi-dimensional HPLC system. The analytical instrument is a three-dimensional HPLC system equipped with three types of separation columns: reversed-phase, anion-exchange, and enantioselective columns. For each dimension, fluorescence detection is carried out (excitation wavelength 470 nm, emission wavelength 530 nm).
Figure 2 shows a flow diagram of the three-dimensional HPLC system. By the reversed-phase column in the first dimension, the target amino acids are separated from other amino acids and interfering substances based on differences in hydrophobicity. After separation, the targeted amino acids are collected online as the mixtures of D- and L-forms, and entire volumes of the collected fractions are injected into the second-dimension anion-exchange column. In this column, the components that eluted together with targeted amino acids from the first-dimension column are separated via electrostatic interaction. The targeted amino acids are again collected online as the mixtures of D- and L-forms, and the entire volumes of the mixtures are injected into the third-dimension enantioselective column. In the third dimension, the D- and L-forms of amino acids are separated and determined. For the first-dimension, a reversed-phase column (Singularity RP18, 1.0 mm ID × 250 mm) is adopted, and the gradient elution using 5-25% aqueous acetonitrile solutions containing 0.025% TFA is carried out. For the second-dimension, a Singularity AX column (1.0 mm ID × 150 mm) is used, and mixed solutions of methanol and acetonitrile (50/50, v/v) containing 0.03% or 0.06% formic acid are selected as the mobile phases. For the third-dimension, a Singularity CSP-001S column (1.5 mm ID × 500 mm) is adopted. For the enantiomer separations of Ala, Val, 2AB, and Nva, a mixture of methanol and acetonitrile (50/50, v/v) containing 0.2% formic acid is used as a mobile phase. For the enantiomer separation of Iva, a mixture of methanol and acetonitrile (90/10, v/v) containing 0.1% formic acid is used. All of these Singularity columns are developed in collaboration with KAGAMI Inc. (Osaka, Japan).
The three-dimensional HPLC system was validated using standard amino acids. The calibration curves showed good linearity (correlation coefficients of 0.9993 or higher) over a concentration range from 5 fmol to 5 pmol per injection. High precision with RSD values in the range 1.33-8.77% was also obtained. For meteorite samples, we obtained high precision with RSD values in the range 3.21-7.84% and accuracy values were in the range 96.6-106.8%, demonstrating that the three-dimensional HPLC technique is applicable to measure chiral amino acids accurately in extraterrestrial samples.
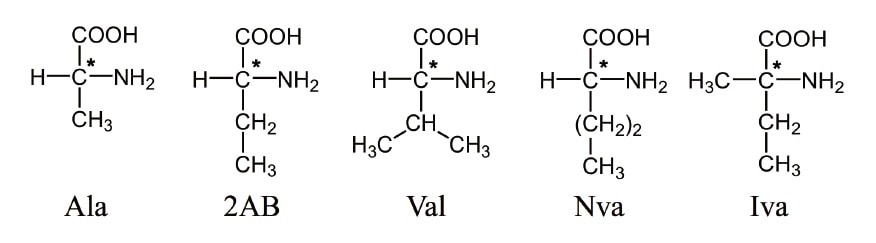
Fig. 1 Structures of target amino acids.
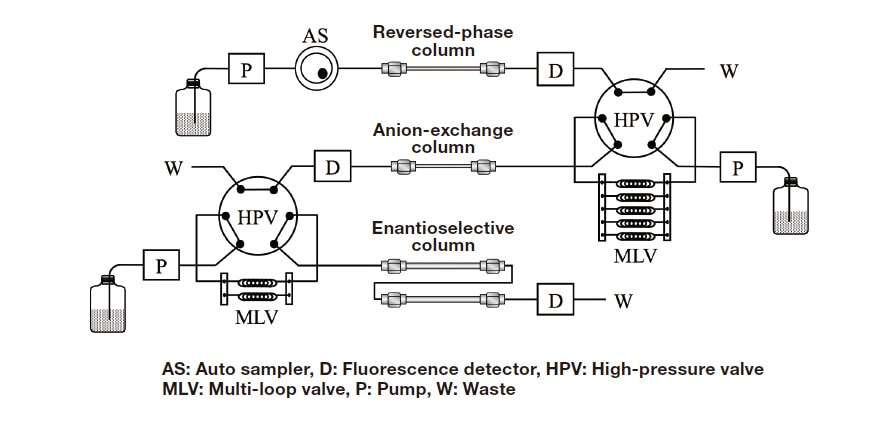
Fig. 2 Flow diagram of the three-dimensional HPLC system.
Organic molecules were extracted from meteorites and other extraterrestrial samples in hot water for 20 h, followed by the hydrolysis at 105°C in 6M aqueous hydrochloric acid for 20 h, then dried under reduced pressure. The residue was re-dissolved in water and neutralized by an aqueous sodium-hydroxide solution. Then, Na-borate buffer and NBD-F were added to yield fluorescent derivatives. After adding an aqueous TFA solution to terminate the reaction, a portion of the reaction mixture was subjected to the three-dimensional HPLC. To avoid contamination of terrestrial amino acids, glassware and vessels were heated at 500°C for 4 h. Figure 3 shows results analyzing the Yamato 002540 meteorite collected in the area of the Yamato mountains in Antarctica. In the results for the reversed-phase separation in the first dimension (Figure 3, upper), a variety of peaks for other substances is observed in addition to peaks for the target amino acids. The fractions for the target amino acids (indicated by solid black underlines in the figure) are collected online, and introduced into the second-dimension. In the results for anion-exchange separation representing the second-dimension (Figure 3, middle), several peaks corresponding to substances other than the targeted amino acids are still observed. The fractions of the target amino acids (indicated by solid black underlines) are again collected online, and introduced into the third-dimension to separate the D- and L-forms of amino acids by the enantioselective column. In the chromatograms of the third-dimension (Figure 3, bottom), the enantiomers of interest are clearly detected with no coexisting components, and the D- and L-forms are present in a roughly 50/50 blend.
Figure 4 shows the chromatograms obtained in the third-dimension enantioselective separation for a sample from the asteroid Ryugu collected by the Hayabusa 2 spacecraft. These results indicate that our technique can clearly identify and determine five amino acids of interest in the Ryugu particles. Concerning the enantiomer ratios, the D- and L-forms of alanine are present in roughly equal proportion; however, the L-form of valine is more abundant than the D-form. This may be due to the contamination of terrestrial amino acids. Although alanine and valine are both proteinogenic amino acids, extraterrestrial samples contain only small quantities of valine, making the effects of contamination non-negligible for this case. The non-proteinogenic amino acids (2-aminobutyric acid, norvaline, and isovaline) were present as almost racemic mixtures with D- and L-forms in roughly equal proportion. The fact that non-proteinogenic amino acids are almost entirely nonexistent on Earth avoids the problem of terrestrial contamination, and allows the accurate analyses of these amino acids reflecting the enantiomer ratios for amino acids in extraterrestrial specimens.
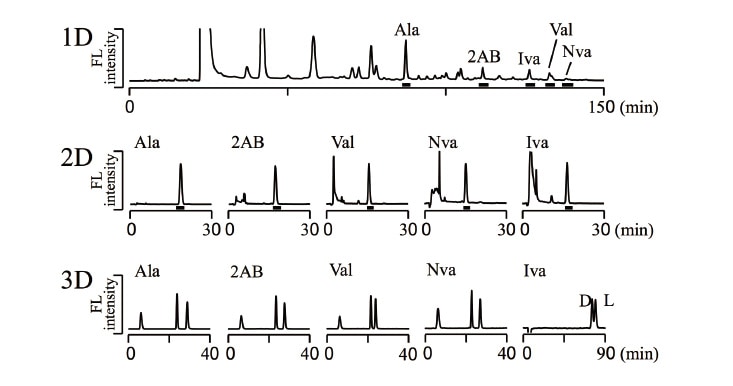
Fig. 3 Three-dimensional HPLC determination of amino acids in the Yamato 002540 meteorite.
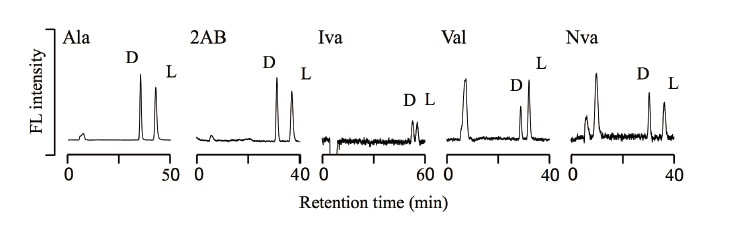
Fig. 4 Determination of amino acid enantiomers in the asteroid Ryugu.
In this work we presented a three-dimensional HPLC technique capable of accurately measuring chiral amino acids in extraterrestrial samples, and the method was applied to analyze the samples collected from the Yamato meteorite and from the asteroid Ryugu. The combination of multiple separation modes allows to achieve extremely high selectivity, and making the three-dimensional HPLC system a powerful tool for analyzing extraterrestrial samples and other complex real-world specimens.
The results obtained for both extraterrestrial samples clearly demonstrate the existence of amino acids in cosmic space. Our findings that such amino acids exist in almost racemic mixtures—with D- and L-forms in roughly 50/50 proportion—suggest that further studies will be needed to clarify the origins of the homochirality observed for amino acids on Earth.
References
C. Ishii, A. Furusho, C.-L. Hsieh, K. Hamase, Multi-dimensional high-performance liquid chromatographic determination of chiral amino acids and related compounds in real world samples, Chromatography, 41, 1-17 (2020).
C. Ishii, K. Hamase, Two-dimensional LC-MS/MS and three-dimensional LC analysis of chiral amino acids and related compounds in real-world matrices, Journal of Pharmaceutical and Biomedical Analysis, 235, 115627 (2023).
A. Furusho, T. Akita, M. Mita, H. Naraoka, K. Hamase, Three-dimensional high-performance liquid chromatographic analysis of chiral amino acids in carbonaceous chondrites, Journal of Chromatography A, 1625, 461255 (2020).
H. Naraoka, K. Hamase, A. Furusho et al., Soluble organic molecules in samples of the carbonaceous asteroid (162173) Ryugu, Science, 379, eabn9033 (2023).