The term carbon neutrality has become ubiquitous in modern discourse. This refers to the goal of reducing carbondioxide(CO2) emissions due to human activity on Earth—ideally, reducing such emissions effectively to zero—to address the problem of climate change caused by CO2 released into Earth's atmosphere through large-scale consumption of fossil-fuel resources. Achieving this goal will require not only reducing use of fossil-fuel resources—and recapturing emitted CO2 for underground burial and eventual reuse, a strategy known as carbon capture, utilization, and storage(CCUS)—but also expanding use of renewable energy sources such as solar and wind power. The various forms of energy we use may be broadly subdivided into two categories: thermal energy, representing around 2/3 of global energy use, and electrical energy, accounting for the remaining 1/3. Thermal energy is used primarily for industrial applications, and, at present, is derived almost entirely from fossil-fuel resources; thus, creating reusable chemical energy to replace existing thermal-energy sources is an urgent priority, and today's most promising candidate strategies for doing so all involve hydrogen. Hydrogen may be used in its base form for systems such as fuel cells or hydrogenpowered turbines, or may be converted into other forms—such as methane, methanol, or ammonia—for use in other ways. If we are to succeed in establishing reusable chemical-energy (thermal-energy) sources without increasing CO2, we will need to use solar power, wind power, and other renewable resources to create hydrogen from water.
At present, the most practical method for doing this is to use electric power obtained from solar, wind, or similar sources to produce hydrogen via electrolysis of water. As is well known, each of the various individual component technologies required for this purpose has already been developed and made available for practical applications. Nonetheless, to date, this method of hydrogen production has not been implemented on a large scale anywhere in the world, for a simple reason: hydrogen produced in this way is considerably more expensive than hydrogen produced from the fossil-fuel resources we use today. Despite the practical availability of the various component technologies, the task of upgrading and combining these technologies to enable ultra-low-cost hydrogen production remains a formidable challenge—and one surely deserving of intense and sustained efforts to address.
In this article I discuss the method of water-splitting using particulate photocatalysts, a technique for producing hydrogen unaccompanied by CO2 generation (known as green hydrogen) from water and solar energy. This approach, which differs from the method described above, is a current focus of hydrogen-production research around the world, but has not yet been deployed in practice. The primary reason for this is that the efficiency of the basic process—in which sunlight excites particulate photocatalysts to generate electron-hole pairs which split water into hydrogen and oxygen—is not yet sufficient for practical applications. However, many researchers agree that, if the technology could be improved to create hydrogen efficiently, it may be capable of producing large quantities of hydrogen at lower cost than the conventional method of solar-cell electrolysis described above. Indeed, the method generates hydrogen and oxygen directly at photocatalyst sites, and if these can be separated from each other we obtain an extremely simple system for producing hydrogen. Of course, it is true that a number of hurdles remain to be overcome—including the development of separation membranes capable of safely separating hydrogen from gaseous hydrogen-oxygen mixtures—but the most important question is how to develop particulate photocatalysts with high efficiency and long photocatalyst lifetimes. In what follows we survey the present state of progress toward this goal and some promising directions for future work.
The discovery that light irradiation of microparticle photocatalysts (with diameters on the order of 1 μm) produces hydrogen and oxygen in a 2:1 stoichiometric ratio was made in 1980 and was reported in three publications that year, including one from my research group. In all cases, the photocatalyst responded only to ultraviolet light, and the quantum yield was on the order of 0.1%. The original photocatalyst microparticles we used were made from strontium titanate (SrTiO3), but a lengthy sequence of improvements over the ensuing years culminated in 2020 with particles achieving nearly 100% (internal) quantum yield, albeit in response to ultraviolet radiation (Figure 1).1) This yield figure indicates that conduction-band electrons and valence-band holes generated by optical excitation are used with nearly 100% efficiency to separate water into hydrogen and oxygen. These photocatalyst particles have sizes in the range 300-500 nm. In brief, electrons and holes generated by light absorption by these particles migrate spontaneously, selectively, and with nearly 100% efficiency to their respective active sites—hydrogen-generation cocatalysts and oxygen generation cocatalysts—without recombining either inside the particle or on its surface. At their respective activation sites, electrons reduce protons (H+) to yield hydrogen, while holes oxidize water molecules (H2O) or hydroxide ions(OH-) to yield oxygen. The reverse reaction—in which the hydrogen and oxygen molecules thus produced recombine into water—does not occur. The driving force responsible for the spontaneous, selective migration of electrons and holes to their respective surface activation sites is believed to be the internal electric field resulting from work-function differences between surface facets, and this hypothesis is supported by evidence from simulations assuming a 0.2 eV gap between the work functions of (100) and (110) crystal planes.
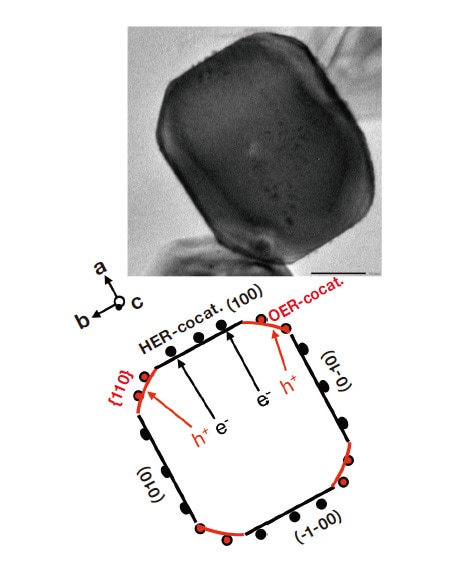
Fig. 1
Above: STEM image of photocatalyst particle.
Below: Schematic diagram indicating cocatalyst orientation, Miller indices of surface facets, and trajectories of electron and hole migration.
From the results of this research, it is crucial that we can achieve quantum efficiency with nearly 100% using particulate photocatalysts with sizes of several hundred nanometers, even without introducing an artificial internal built-in electric field like that in the p-n junctions of solar cells. Moreover, we have found that water can be efficiently hydrolyzed by using nanosilica to form a fixed sheet of these high-efficiency photocatalyst particles on a glass substrate, then adding a 100-μm layer of distilled (electrolyte-free) water to form a water-splitting panel. We have assembled 1600 of these water-splitting panels, each of dimensions 25 cm × 25 cm, to form a solar-hydrogen-production system with a total light-acceptance area of 100 m2 (Figure 2), and we have demonstrated that this system, equipped with hydrogen separation membranes, is capable of producing hydrogen in practice.2)
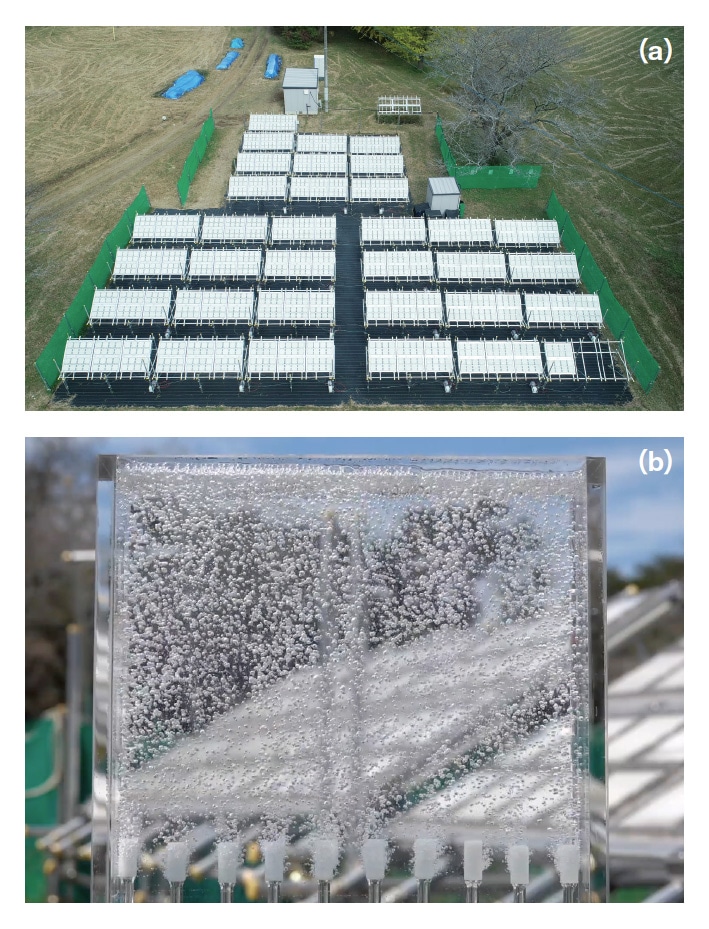
Fig. 2
(a) 100 m2 water-splitting panel.
(b) Gaseous hydrogen-oxygen mixture produced by water-splitting panel.
This experiment serves as a proof-of-concept demonstration that particulate photocatalysts can be used to produce solar hydrogen (green hydrogen); however, the hydrogen-production efficiency of this system remains low. The primary reason for this is that the photocatalyst we use, aluminum-doped SrTiO3, only absorbs ultraviolet light; thus, despite achieving a quantum yield of nearly 100%, its solar-energy conversion efficiency is less than 1%. In addition, the water-splitting panels we have fabricated, as well as our overall 100-m2 hydrogen production and separation system, are costly and cannot be used to produce inexpensive hydrogen. Thus, we will need to revisit each of the individual component technologies. Among the various hurdles to overcome, the most urgent imperative is to increase the solar-energy conversion efficiency, which must be at least 5%, and should ideally be around 10%, for a practically viable system. Thus the most pressing challenge for realizing green hydrogen-production systems is the development of photocatalysts with high solar-energy conversion efficiency.
Achieving high solar-energy conversion efficiency requires developing photocatalysts that respond to visible light. In particular, to convert solar energy with an efficiency on the order of 10% we will need photocatalyst materials with absorption-edge wavelengths of 600 nm or longer. In fact, we have already discovered several candidate photocatalyst materials with absorption edges in the range 600-700 nm and the potential to exhibit water-splitting capabilities. For some of these candidates, we have further confirmed the ability to hydrolyze water, yielding hydrogen and oxygen in a 2:1 ratio, using light at these longer wavelengths; however, one problematic aspect is that quantum yields for this hydrolysis remain at a level of 1% or lower.
Why is it so difficult to increase the quantum yield for photocatalysts capable of long-wavelength absorption? One factor is that such photocatalysts must necessarily have small band-gap energies, whereupon the energies of electron-hole pairs produced by light absorption are correspondingly low. Therefore, it is necessary to effectively lower the activation-energy barrier for hydrogen and oxygen production. Another factor is that visible-light-sensitive photocatalysts tend to have complex structures, with sulfur or nitrogen among their constituent elements; this makes it difficult to fabricate photocatalyst microparticles with few defects. Overcoming this hurdle will require shrinking catalyst particles to sizes of 100 nm or below—perhaps to sizes of just a few tens of nanometers. Precision control of cocatalysts locations will then become important as well.
In developing photocatalyst materials to satisfy these conditions, methods for experimentally characterizing photocatalyst particles will play an essential role. In particular, STEM and SEM for structural analysis, and EDX and EELS for analyzing ultra-miniature sample regions, are extremely powerful tools. We are hopeful that these techniques, together with laser-based spectroscopic analysis and other methods for characterizing photocatalysts at the atomic level and correlating those findings with observations of photocatalytic activity, will enable us to develop new photocatalysts with the specific properties we seek.
References