Automation of Pretreatment by Column-Switching
The analysis of biological specimens and samples containing large quantities of impurities requires pretreatment, such as removal of the impurities and the condensation of the sample.
Pretreatment, exerting a significant impact on the results of the analysis and being a critical element, to a large extent depends on the experience and skill levels of the individual performing the analysis.
Therefore, reliable pretreatment operations are of paramount importance.
Among the processes that are widely employed in pretreatment are solid-phase extraction and column-switching.
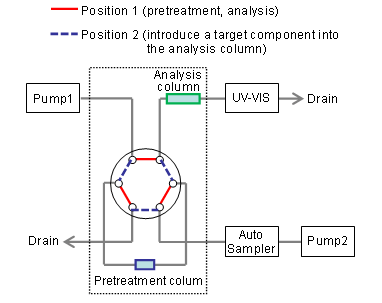
The Chromaster system permits the automation of pretreatment through the use of column-switching combined with the use of optional valves.
The following is an example of analysis and evaluation of drugs found in blood serum.
Analysis of phenytoin in blood by column-switching
Sample
Standard phenytoin reagent added to human serum.
(the sample was centrifuged, and the supernatant was used as an injection specimen)
Purpose
To study the automation of pretreatment for phenytoin analysis.

* Given that phenytoin is toxic from 10 to 20 µg/mL(effective concentrationinblood), and 20 µg/mL or higher in in-blood concentration, it has been verified that the method employed in this procedure can accurately determine the amount of phenytoin in blood.
Configuration of the system employed
5110 pump x2
5210 Autosampler
5310 Column Oven (with a 6-way 2-position valve)
5420 UV-VIS Detector
Conditions
Analysis column
| Mobile phase | (A) 50 mM KH2PO4-K2HPO4(pH6.9)/CH3CN=95/5 (B) CH3CN *(A)/(B) = 65/35 |
|---|---|
| Flow rate | 1.0 mL/min |
| Column | HITACHI LaChrom C18 (5 µm) (4.6 mm I.D. x 150 mm) (P/N: 891-5050) |
| Column Temperature | 40°C |
| Detection | 210 nm |
Pretreatment column
| Mobile phase | (A) 50 mM KH2PO4-K2HPO4(pH6.9)/CH3CN = 95/5 (B) CH3CN *(A)/(B) = 95/5 |
|---|---|
| Flow rate | 1.0 mL/min |
| Injection vol. | 20 µL |
| Column | Mspak PK-4A(4.0 mmI.D. x 10 mm, Shodex)*1 |
| Column Temperature | 40°C |
*1:This is a pretreatment column for column-switching analysis; it is designed to retain low-molecular weight components only, without retaining protein.

Evaluation of a column-switching pretreatment method
The analysis of phenytoin(anti-epilepsy agent) in blood requires the removal of protein and other components. In view of this fact, we studied a method of exclusively introducing the target component, phenytoin, into the analysis column by removing protein through the use of a pretreatment column.

Evaluation of the conditions for the pretreatment column (1)
Purpose

To verify the conditions under which protein is eluted
Conditions
Pretreatment column
| Mobile phase | (A) 50 mM KH2PO4-K2HPO4(pH6.9)/CH3CN=95/5 (B) CH3CN *(A)/(B) = 95/5 |
|---|---|
| Flow rate | 1.0 mL/min |
| Injection vol. | 20 µL |
| Column | Mspak PK-4A (4.0 mmI.D. x 10 mm, Shodex) |
| Column Temperature | 40°C |
Results
It was verified that proteins are not retained in the column.
Evaluation of a column-switching pretreatment method (2)
Purpose
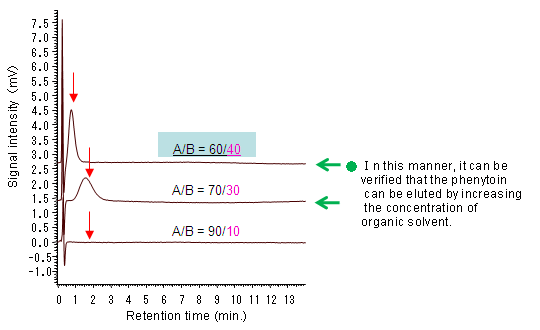
To verify the conditions under which the target component (phenytoin) is introduced into an analysis column.
The evaluation was conducted under the following conditions: phenytoin standard, 10 mg/L (injection volume: 20 µL); detection: 254 nm.
Conditions
Pretreatment column
| Mobile phase | (A) 50 mM KH2PO4-K2HPO4(pH6.9) (B) CH3CN |
|---|---|
| Flow rate | 1.0 mL/min |
| Column | Mspak PK-4A (4.0 mmI.D. x 10 mm, Shodex) |
| Column Temperature | 40°C |
Evaluation of analysis column condition
Purpose
To verify the conditions under which the target component (phenytoin) is retained in the analysis column and separated.
The evaluation was conducted under the following conditions: phenytoin standard, 10 mg/L (injection volume: 20 µL); detection: 254 nm.

Conditions
Pretreatment column
| Mobile phase | (A) 50 mM KH2PO4-K2HPO4(pH6.9) (B) CH3CN |
|---|---|
| Flow rate | 1.0 mL/min |
| Injection vol. | 20 µL |
| Column | HITACHI LaChrom C18 (5 µm) (4.6 mmI.D. x 150 mm) |
| Column Temperature | 40°C |
Results
Phenytoin was introduced from the pretreatment column to the analysis column.
The analysis condition was designated as A/B = 60/40.
Evaluation of valve-switching time
-
Introduce serum into a pretreatment column; verify the amount of time required for protein removal (protein detected at 280 nm).
- For these tests, the valve-switching time was set to 5 minutes. - The concentration of the mobile phase was determined based upon the results of the previous pretreatment column condition evaluation.
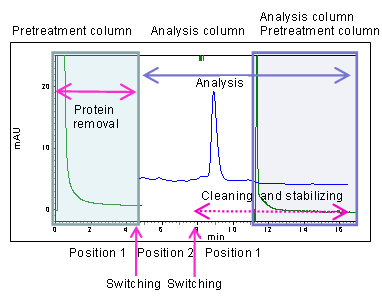
Results of analysis by column-switching
Sample
A standard phenytoin sample (10 µg/mL) added to human serum
* Supernatant injected after centrifuge separation


The rate of recovery of phenytoin by the automation of pretreatment was 85.3%. At 10 µg/mL, the reproducibility of elution time was CV: 0.04%, and the reproducibility of the area under the curve was CV: 1.91%.Given that the toxic region of phenytoin is 10 to 20 µg/mL in effective concentration in blood, and 20 µg/mL or higher in in-blood concentration, it has been verified that the method employed in this procedure can accurately determine the amount of phenytoin in blood.
NOTE:
These data are an example of measurement; the individual values cannot be guaranteed.
The system is for research use only, and is not intended for any animal or human therapeutic or diagnostic use.
In order to read a PDF file, you need to have Adobe® Reader®
