Analysis of Combination Drugs (Identification and Quantitative Analysis by DAD)
Prescription drugs generally contain a single type of active pharmaceutical ingredient (API). However, the number of combination drugs containing more than two types of APIs has been increasing in recent years. Benefits of combination drugs include the reduced number of medications and the prevention of missed doses. Presented here is the analysis of a combination drug containing two types of APIs by HPLC/DAD(diodearrayde tector).
Analysis Example of Standard Sample

AnalyticalConditions
| Column | HITACHI LaChrom C18 (5 µm) (4.6 mm I.D. x 150 mm) |
|---|---|
| Eluent | Phosphoric acid buffer/Methanol (a)30/70 (b)10/90(v/v), (a)(b)Gradient |
| Flow rate | 1.0 mL/min |
| Column temperature | 25°C |
| Detection wavelength | DAD 220 - 400 nm (237 nm, 254 nm)* |
| Injection vol. | 20 µL |
* The wavelength specified in the Pharmacopoeia was used for the detection of each component.
Linearity of Standard Sample (8 - 80 mg/L each)

Reproducibility of Standard Sample
| Retention time(% RSD) | Area value (% RSD) | |
|---|---|---|
| A | 0.06 | 0.27 (237 nm) |
| B | 0.04 | 0.18 (254 nm) |

A good linearity was obtained for both components A and B over the concentration range of 8 - 80 mg/L.
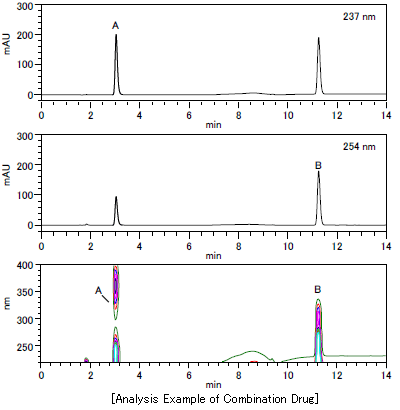
Analysis Example of Combination Drug
Preparation Method for Combination Drugs

Quantitative Value for Combination Drug
| Result of Quantitative Analysis | |
|---|---|
| A | 34.11 mg/L |
| B | 39.24 mg/L |
Identification based on Spectrum
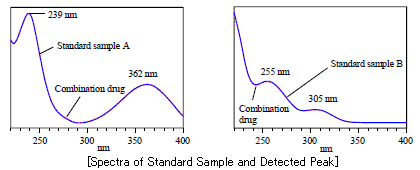
DAD allows for confirmation of the maximum absorption of the absorption spectrum for each component.
In the section of Liquid Chromatography under the General Tests of the Japanese Pharmacopoeia Sixteenth Edition, it is stated "If a detector which is able to obtain chemical structural information of the component at the same time is used, highly specific identification can be achieved by confirming identity of the chemical structure of the component and that of an authentic specimen, in addition to the identity of their retention times." This statement suggests the use of the LC/DAD for identification.
The preparation of the draft for the Japanese Pharmacopoeia Seventeenth Edition is currently in progress and the rationalization which allows for performing the identification by using the highly specific chromatography for quantitative analysis method is also being considered as a mean for the "rationalization for identification."*1 *2
*1Guideline for Drafting the Japanese Pharmacopoeia, Seventeenth Edition (Draft)
*2Opinions, etc. received for "Opinion Survey Concerning Guideline for Drafting the Japanese Pharmacopoeia, Seventeenth Edition Draft (Draft)" (April, 2012)
* The Japanese Pharmacopoeia does not specify the test methods for combination drugs.
* This analytical sample was provided by Division of Physical Pharmaceutical Chemistry, Faculty of Pharmacy, Keio University.
NOTE:
These data are an example of measurement; the individual values cannot be guaranteed.
The system is for research use only, and is not intended for any animal or human therapeutic or diagnostic use.
In order to read a PDF file, you need to have Adobe® Reader®
